Return To The Producets List
ProteanFect™ Max Transfection Kit
ProteanFect™ Max Transfection Kit is designed to efficiently transfect a wide range of primary cells with high efficiency, while maintaining an excellent safety profile. It facilitates easy scale-up and meets the demands of high-throughput experiments. Additionally, it is user-friendly, making transfection of primary cells as straightforward as transfecting HEK293T cells. For research use only.
| Number | Standard | Price(USD) | Stock |
|---|---|---|---|
| PT02-0010B | 0.1 mL | 630 | In 4 weeks |
| PT02-0020B | 0.2 mL | 1056 | In 4 weeks |
| PT02-0050B | 0.5 mL | 2313 | In 4 weeks |
| PT02-0100B | 1 mL | 3878 | In 4 weeks |
| PT02-0500B | 5 mL | 16677 | In 4 weeks |
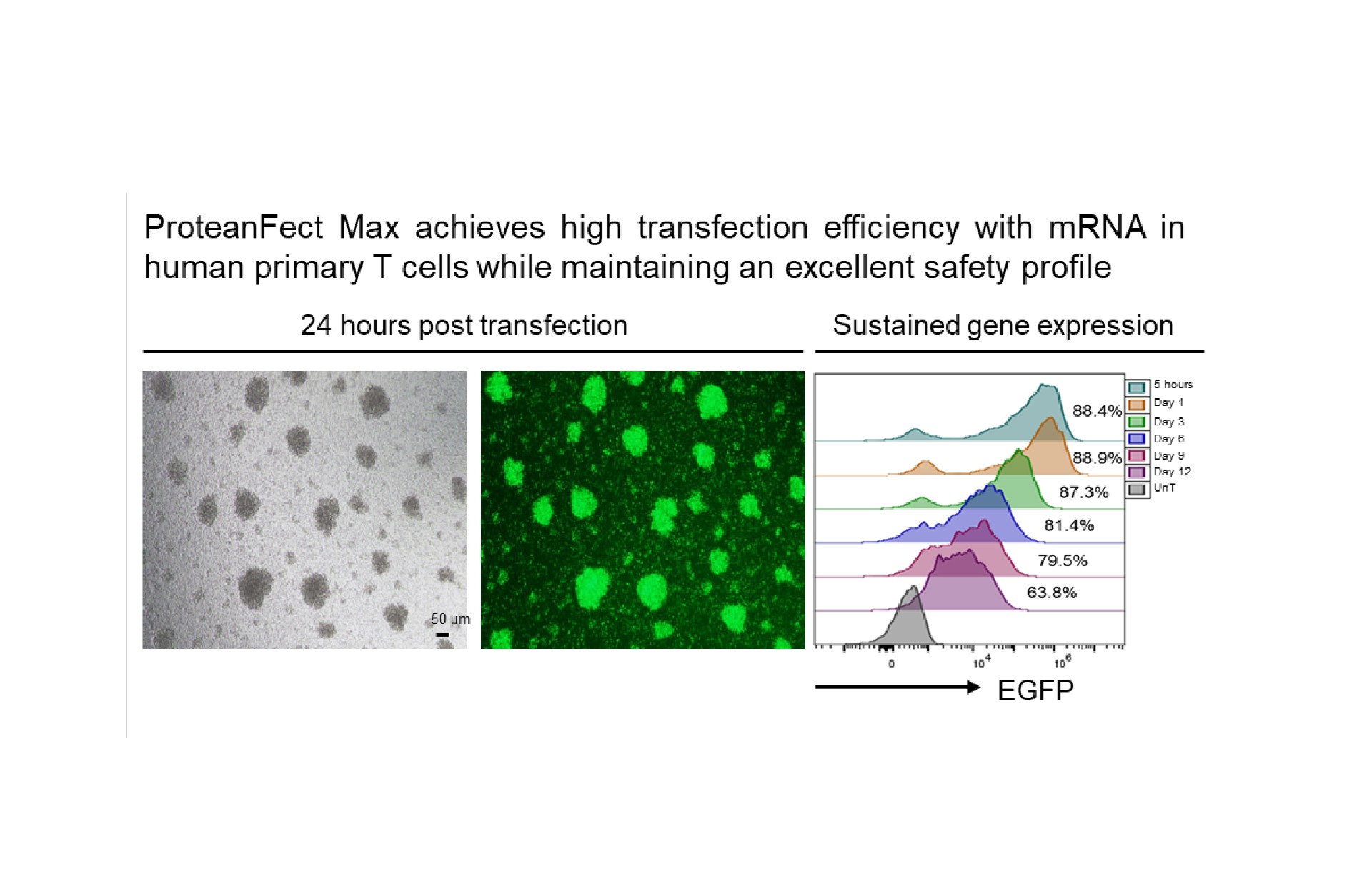
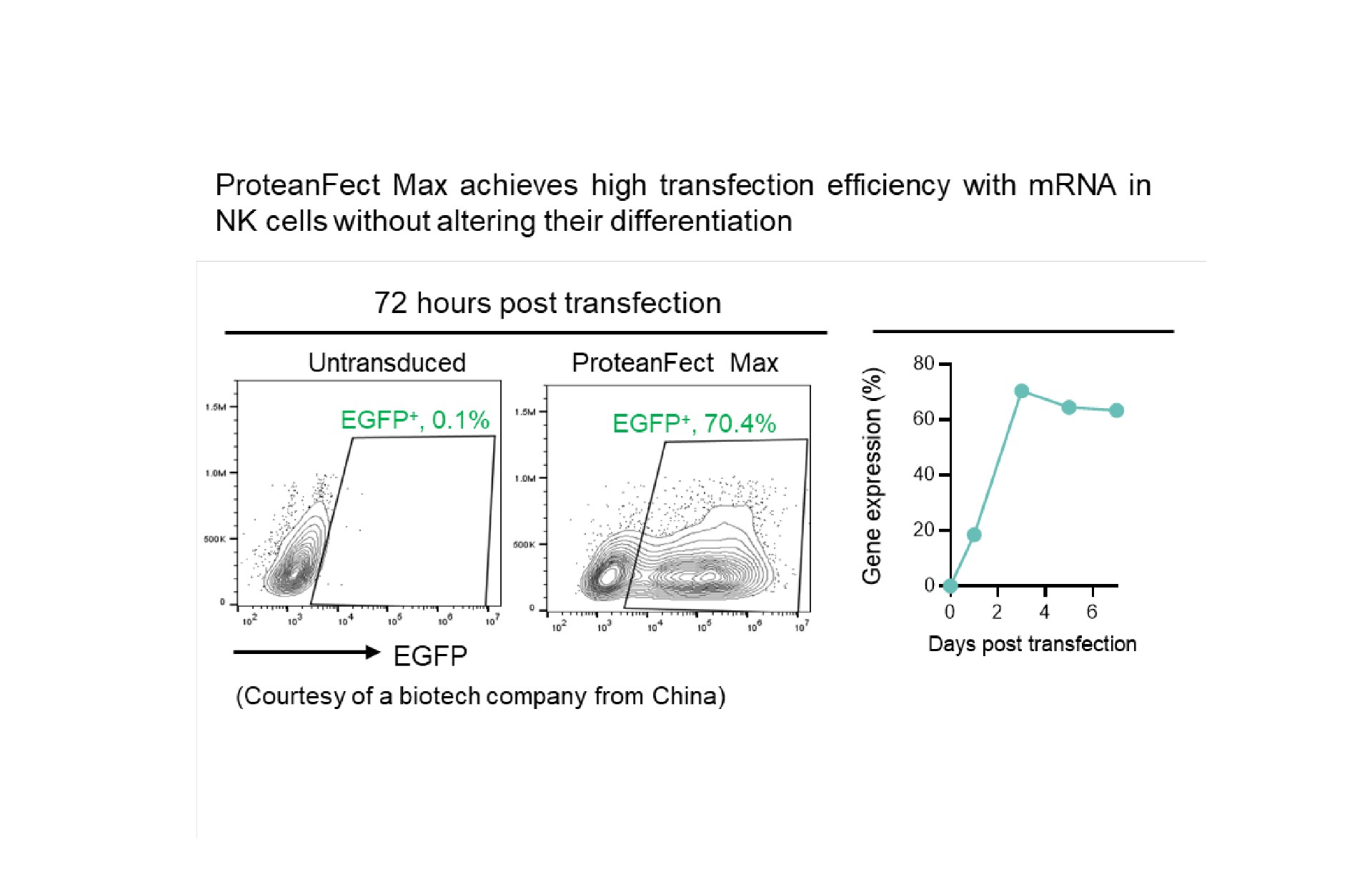
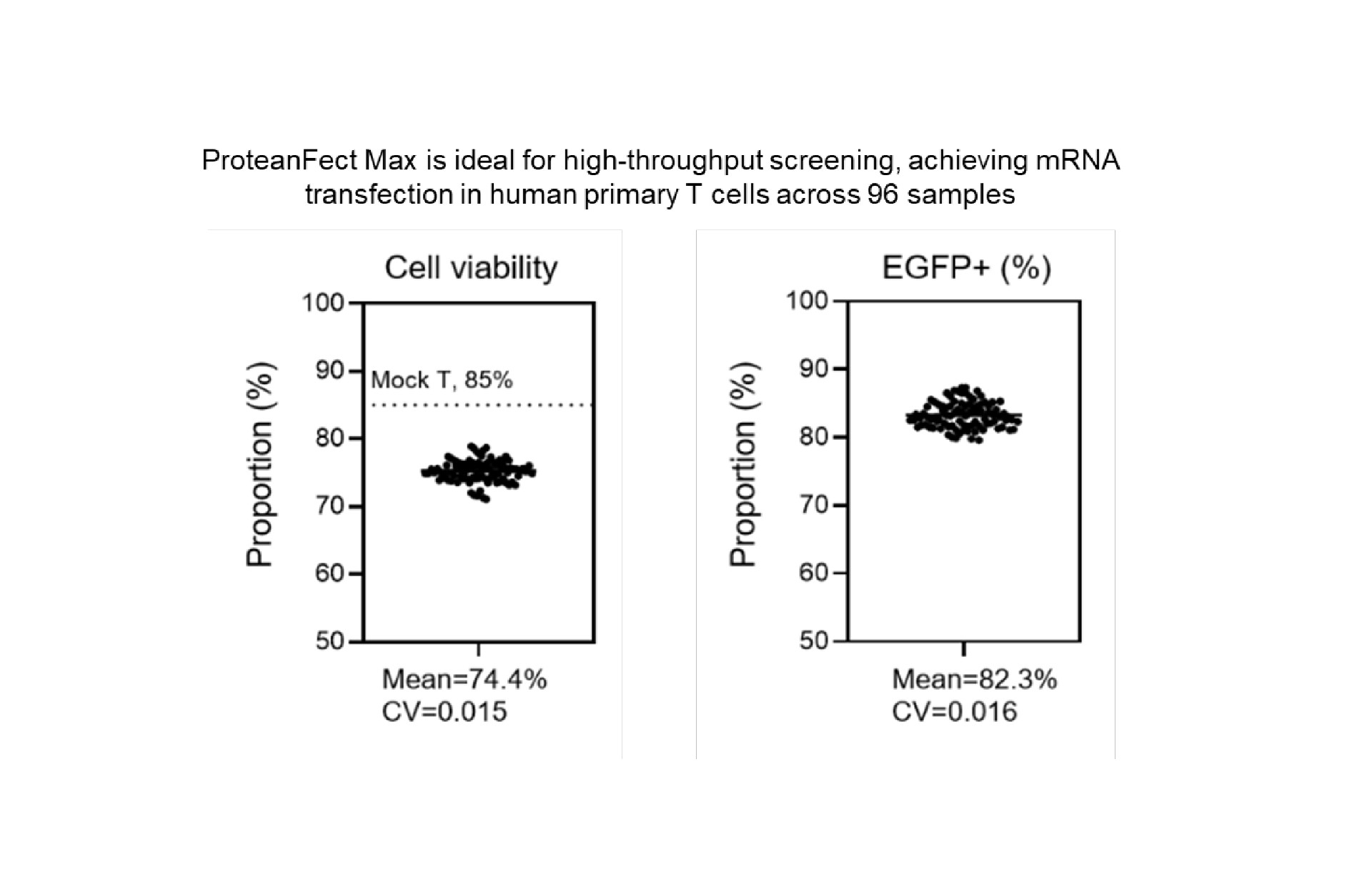
Transfection Performance
Product Information
Components & Storage
The kit is shipped on dry ice. Once received, store the components as indicated below. The kit includes positive control samples with EGFP-encoding mRNA and plasmid DNA (pDNA) to verify transfection efficiency.
| Components | Storage |
|---|---|
| Reagent A | 2-8°C |
| Reagent B | -20°C(Avoid repeated freeze-thaw cycles) |
| Reagent C | 2-8°C |
| EGFP mRNA (1 µg/µL) | -80°C (Avoid repeated freeze-thaw cycles) |
| GFP pDNA (0.5 µg/µL) | -20°C (Avoid repeated freeze-thaw cycles) |
Shipping Information
Shipped on Dry Ice
Compatible Cell Lines
Primary Cells and Cell Lines Successfully Transfected Using ProteanFect™ Max Transfection Kit
| Cell lines | Tested Nucleic Acid Types for Transfection |
|---|---|
| Human primary T cells | mRNA, siRNA |
| Human primary NK cells | mRNA, siRNA |
| Human primary monocytes | mRNA, siRNA |
| Human primary CD34+ hematopoietic stem cells | mRNA, siRNA |
| Mouse primary cardiomyocytes | mRNA, siRNA |
| Mouse primary neurons | pDNA, mRNA, siRNA |
| Mouse primary glial cells | pDNA, mRNA, siRNA |
| Mouse primary T cells | mRNA, siRNA |
| Mouse primary NK cells | mRNA, siRNA |
| Large yellow croaker primary mesenchymal stem cells | mRNA, siRNA |
| Jurkat T (human T lymphoblastic leukemia cells) | pDNA, mRNA, siRNA |
| LX-2 (human hepatic stellate cells) | pDNA , mRNA, siRNA |
| HepG2 (human liver tumor cells) | pDNA, mRNA, siRNA |
| THP-1 (human acute monocytic leukemia cell line) | mRNA, siRNA |
| Raji (human Burkitt lymphoma cells) | mRNA, siRNA |
| K562 (human chronic myeloid leukemia cells) | pDNA, mRNA, siRNA |
| MOLT-16 (human T lymphoblastic leukemia cells) | mRNA, siRNA |
| SH-SY5Y (human neuroblastoma cells) | pDNA, mRNA, siRNA |
| U2OS (human osteosarcoma cells) | pDNA, mRNA, siRNA |
| HFF (human foreskin fibroblasts) | mRNA, siRNA |
| HEK-293 (human embryonic kidney cell line) | pDNA, mRNA, siRNA |
| MC38 (mouse colon cancer cells) | mRNA, siRNA |
| RAW264.7 (mouse mononuclear macrophage leukemia cells) | mRNA, siRNA |
| LLC (mouse Lewis lung cancer cells) | pDNA, mRNA, siRNA |
| C2C12 (mouse myoblasts) | pDNA, mRNA, siRNA |
| COS7 (African green monkey kidney fibroblast-like cells) | pDNA, mRNA, siRNA |
Product Data
Transfection Protocol for mRNA per Well of a 96-Well Plate
| Scheme | Step | Cell Line Transfection Protocol for mRNA per Well of a 96-Well Plate |
|---|---|---|
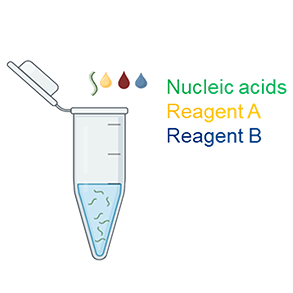 |
1. Transfection Complex Preparation |
1.1 Mix Reagent A with mRNA Mix 0.5 µg of mRNA with 40 µL of Reagent A. Note: Invert Reagent A briefly before use to ensure uniformity. 1.2 Add Reagent B Add 1.4 µL of Reagent B to the mixture. Mix gently by pipetting up and down 20-30 times or vortexing for 10 seconds. |
 |
2. Cell Preparation |
2.1 Suspension cells Harvest the cells by centrifugation at 300 g for 5 minutes. Discard the supernatant and wash cells once with Opti-MEM. Resuspend cells with Opti-MEM and adjust concentration to 5×10⁶ - 1×10⁷ cells/mL. Note: Avoid including FBS in the transfection medium. 2.2 Adherent cells Maintain 50%-80% cell confluence. Remove medium, wash cells once with Opti-MEM, then add 20 µL of Opti-MEM. Note: Avoid including FBS in the transfection medium. Optional: Harvest cells by trypsinization, then resuspend them in Opti-MEM at a concentration of 5×10⁶ - 1×10⁷ cells/mL for subsequent transfection. |
 |
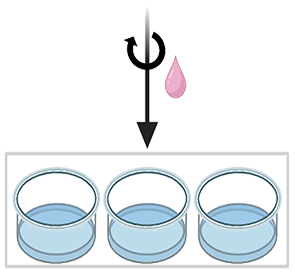
3. Transfection |
3.1 Mix complex with cells For suspension cells, mix 40 µL of transfection complex with 20 µL of cell suspension and gently pipet up and down 2-3 times. For adherent cells, apply directly to the cells. 3.2 Incubation Incubate the cells with the transfection complex for 45-60 minutes in a cell culture incubator. 3.3 Termination Terminate the reaction by adding ≥200 µL of culture medium (10X cell suspension), centrifuge at 300 g for 5 minutes, and discard the supernatant. For adherent cells, replace the transfection mixture with ≥200 µL of culture medium (10X cell suspension). Note: The cell pellet may adhere to the tube walls. Gently remove the supernatant to minimize cell loss. 3.4 Post-transfection culture Incubate transfected cells in culture medium and assess transfection efficiency after 5 to 48 hours, or at an appropriate time. |
| Scheme | Step | Primary Cell Transfection Protocol for mRNA per Well of a 96-Well Plate |
|---|---|---|
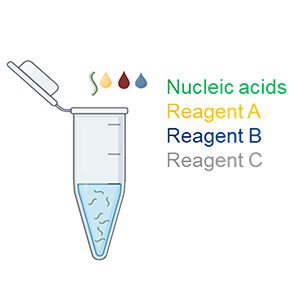 |
1. Transfection Complex Preparation |
1.1 Mix Reagent A with mRNA Mix 0.5 µg of mRNA with 40 µL of Reagent A. Note: Invert Reagent A briefly before use to ensure uniformity. 1.2 Add Reagent B Add 0.7 µL of Reagent B to the mixture. Mix gently by pipetting up and down 20-30 times or vortexing for 10 seconds. 1.3 Add Reagent C Add 8 µL of Reagent C to the mixture. Mix gently by pipetting up and down 2-3 times or vortexing for 2-3 seconds. |
 |
2. Cell Preparation |
2.1 Suspension cells Harvest the cells by centrifugation at 300 g for 5 minutes. Discard the supernatant and wash cells once with Opti-MEM. Resuspend cells with Opti-MEM and adjust concentration to 5×10⁶ - 1×10⁷ cells/mL. Note: Avoid including FBS in the transfection medium. 2.2 Adherent cells Maintain 50%-80% cell confluence. Remove medium, wash cells once with Opti-MEM, then add 20 µL of Opti-MEM. Note: Avoid including FBS in the transfection medium. Optional: Harvest cells by trypsinization, then resuspend them in Opti-MEM at a concentration of 5×10⁶ - 1×10⁷ cells/mL for subsequent transfection. |
 |
3. Transfection |
3.1 Mix complex with cells For suspension cells, mix 40 µL of transfection complex with 20 µL of cell suspension and gently pipet up and down 2-3 times. For adherent cells, apply directly to the cells. 3.2 Incubation Incubate the cells with the transfection complex for 15-30 minutes in a cell culture incubator. 3.3 Termination Terminate the reaction by adding ≥200 µL of culture medium (10X cell suspension), centrifuge at 300 g for 5 minutes, and discard the supernatant. For adherent cells, replace the transfection mixture with ≥200 µL of culture medium (10X cell suspension). Note: The cell pellet may adhere to the tube walls. Gently remove the supernatant to minimize cell loss. 3.4 Post-transfection culture Incubate transfected cells in culture medium and assess transfection efficiency after 5 to 48 hours, or at an appropriate time. |
Technical FAQ
1.1 Optimize Transfection Parameters
Optimize transfection parameters for each cell type. Extended incubation time: Adjust the incubation time of the transfection complex with cells. The maximum incubation time is 2 hours for cell lines, and 30 minutes for primary cells. Increase ProteanFect™ transfection complex: Consider increasing the amount of transfection complex to improve transfection efficiency.
1.2 Severe Cytotoxicity Caused by Plasmid DNA
The transfection of pDNA into primary cells, such as primary T cells, can induce cytotoxicity and inflammatory responses. Due to the risk of significant toxicity, pDNA transfection is generally not recommended for primary T cells.
1.3 Improve Plasmid DNA Quality
Transfection efficiency is highly dependent on the quality of the plasmid DNA. Endotoxin-free: Use an endotoxin-free kit to prepare plasmid DNA. Optimal concentration: Ensure an OD260/280 ratio between 1.7 and 1.9, and dilute DNA to 0.5–2 µg/µL using nuclease-free water.
1.4 Improve Cell Condition
For cell lines, transfect cells with >90% viability, confirmed by trypan blue exclusion. Avoid using cells beyond 15 passages, and allow 2-3 passages for recently thawed cells to stabilize before transfection.
For primary cells, proper activation is crucial for optimal transfection efficiency. For example, human primary T cells generally achieve the best transfection results after stimulation with anti-CD3/CD28 activation beads or antibodies for 2-10 days, with peak efficiency typically observed around days 4-6.
1.5 Use Positive Control
We recommend using a 96-well plate format to optimize transfection conditions for a specific cell type, with EGFP mRNA as the positive control.
Optimize transfection parameters for each cell type. Extended incubation time: Adjust the incubation time of the transfection complex with cells. The maximum incubation time is 2 hours for cell lines, and 30 minutes for primary cells. Increase ProteanFect™ transfection complex: Consider increasing the amount of transfection complex to improve transfection efficiency.
1.2 Severe Cytotoxicity Caused by Plasmid DNA
The transfection of pDNA into primary cells, such as primary T cells, can induce cytotoxicity and inflammatory responses. Due to the risk of significant toxicity, pDNA transfection is generally not recommended for primary T cells.
1.3 Improve Plasmid DNA Quality
Transfection efficiency is highly dependent on the quality of the plasmid DNA. Endotoxin-free: Use an endotoxin-free kit to prepare plasmid DNA. Optimal concentration: Ensure an OD260/280 ratio between 1.7 and 1.9, and dilute DNA to 0.5–2 µg/µL using nuclease-free water.
1.4 Improve Cell Condition
For cell lines, transfect cells with >90% viability, confirmed by trypan blue exclusion. Avoid using cells beyond 15 passages, and allow 2-3 passages for recently thawed cells to stabilize before transfection.
For primary cells, proper activation is crucial for optimal transfection efficiency. For example, human primary T cells generally achieve the best transfection results after stimulation with anti-CD3/CD28 activation beads or antibodies for 2-10 days, with peak efficiency typically observed around days 4-6.
1.5 Use Positive Control
We recommend using a 96-well plate format to optimize transfection conditions for a specific cell type, with EGFP mRNA as the positive control.
Transfected cells may exhibit transient changes in behavior, but typically, viability will be restored by the second day post-transfection.
In 96-well formats, it is common for the pellet to be less distinct and may adhere to the tube walls. Gently pipetting can help minimize cell loss.
For further questions, please contact us at: tech@nanoportalbio.com.